- Received March 01, 2024
- Accepted April 24, 2024
- Publication June 11, 2024
- Visibility 36 Views
- Downloads 0 Downloads
- DOI 10.18231/j.ijohd.2024.020
-
CrossMark
- Citation
Comparative analysis of chlorhexidine and listerine mouthwashes on buccal cell health: A clinical and cytological investigation
Introduction
Dental plaque is prevented by both mechanical (like brushing) and chemical (like mouth rinses) methods. However, inadequate technique or patient noncompliance affects the mechanical methods. Chlorhexidine mouthwash (CHX) is a widely used mouthwash due to its efficacy in inhibiting plaque. It acts by altering bacterial cell permeability. Despite its effectiveness, CHX has side effects like staining, taste alteration and some studies shows genotoxic effects of chlorhexidine on buccal cells as well.[1] Listerine is another common mouthwash, endorsed by the American Dental Association for its plaque and gingivitis control.[2] As it has herbal components and it is used commonly; in the present study this mouthwash is assessed along with chlorhexidine.
In the present study we’re monitoring genotoxic effects of the two most commonly used mouthwashes on buccal mucosa through biomarkers like micronucleus assay in exfoliated oral cells. This will provide insights into potential carcinogenic risks from this mouthwash, which is crucial in understanding oral carcinogenesis.[3]
Aims and Objectives
To compare and assess the genotoxic effects of Chlorhexidine mouthwash and Listerine mouthwash on exfoliated buccal cells with utilization of the micronucleus test.
Objective
To assess the clinical and genotoxic effects of Chlorhexidine mouthwash on gingival bleeding, utilizing the gingival index measured from baseline to 3 weeks.
To assess the clinical and genotoxic effects of Listerine mouthwash on gingival bleeding, employing the gingival index measured from baseline to 3 weeks.
To compare the clinical and genotoxic impacts of Chlorhexidine mouthwash and Listerine mouthwash on gingival bleeding by analyzing the gingival index from baseline to 3 weeks.
Materials and Methods
The study was done in the Department of Periodontology, K.M. Shah Dental College and Hospital, Sumandeep Vidyapeeth, Vadodara, Gujarat.
The study was started after IEC approval and the study duration was 3 months. The subjects were selected from the out-patient department.
Selected participants were in the age group of 18 to 60 years. Participants, who were willing and able to read, understand English and sign the informed consent form, were systemically healthy, and had gingivitis were selected.
Pregnant female patients or lactating mothers, patients having allergic reactions or hypersensitivity to any product used in the study, individuals who have a habit of smoking & tobacco chewing, participants not willing to participate in the study and further follow-up, participants who had undergone periodontal surgery in past 6 months and the participants who had used antibiotics and anti-inflammatory in the last 3 months were excluded from the study.
Study design
This is a Parallel study with two groups.
Sample size calculations
According on the study done by Erdemir EO et al.[4] minimum 35 samples required for present study. To overcome the non-response error the final sample size was increased to 40 (20 per group).
n 1 = NZ2 P (1-P)d 2 (N-1) + Z2 P (1-P)Where, n1 = sample size with finite population correction N = population size, Z = Z statistic for level of confidence, P = expected population (if prevalence is 20%, P = 0.2), D = precision (if the precision is 50%, than d = 0.05).
A total of 40 study subjects were assigned into two groups using the coin flip method. Group A (20): Use of CHX mouthwash after phase 1 periodontal therapy. Group B (20): Use of Listerine mouthwash after phase I periodontal therapy. After the treatment, the participants were asked to gargle with the allotted mouthwash for 30 sec twice daily for 3 weeks. Gingival Index (GI) parameters was recorded on 1st day (baseline) and at 3rd week. Oral cells were collected on days 1 and at the end of 3 weeks, fixed, mounted on slides, observed under light microscope (×40/×100) for calculation of micronucleated cells. Micronuclei were counted using Tolbert’s criteria[5] by a masked oral pathologist.
|
Parameters For Cell Inclusion in the Cells to be Scored: |
The suggested criteria for identifying MN are |
|
1. Intact cytoplasm and relatively flat cell position on the slide; 2. Little or no overlap with adjacent cells; 3. Little or no debris; 4. Nucleus normal or intact, nuclear perimeter smooth and distinct. |
1. Rounded smooth perimeter suggestive of a membrane; 2. Less than a third the diameter of the nucleus but large enough to discern shape and colour; 3. Feulgen positive i.e. pink in bright film illumination 4. Staining intensity similar to that of the nucleus; 5. Texture similar to that of the nucleus; 6. Same focal plane as nucleus; 7. Absence of overlap with or bridge to the nucleus. |
Statistical analysis
Results were analyzed using Descriptive and Inferential methods. The Independent t-test compared groups, while paired t-tests assessed within-group differences.
Results
This comparative clinical study aimed to assess and compare the clinical and genotoxic effects of Chlorhexidine mouthwash and Listerine mouthwash on buccal cells. Parameters assessed were Gingival Index and Cytologic Parameters i.e. micronucleated cells and micronuclei count at baseline and 3 weeks.
Within the Chlorhexidine mouthwash (CHX) group, there was a significant decrease in GI from baseline to 3 weeks (mean difference = 1.335, p < 0.001). Similarly, micronuclei count increased significantly from baseline to 3 weeks (mean difference = 2.1, p < 0.001), along with a higher count of micronucleated cells (mean difference = 3.55, p < 0.001). ([Table 1], [Figure 2], [Figure 3]) In the Listerine group, there was also a notable decrease in GI from baseline to 3 weeks (mean difference = 1.525, p < 0.001), accompanied by a significant increase in micronuclei count (mean difference = 0.55, p < 0.001) and micronucleated cells (mean difference = 0.6, p < 0.001). ([Table 3], [Figure 4], [Figure 5])
Comparison between the two groups at baseline showed no significant differences in GI, micronuclei count, or micronucleated cells, indicating similar baseline conditions. However, by the 3 weeks, the Chlorhexidine mouthwash group demonstrated significantly higher micronuclei (p < 0.001) and a higher count of micronucleated cells (p < 0.001) compared to the Listerine group. ([Table 4], [Figure 1])
|
Parameters |
N |
Mean |
Std. Deviation |
Paired Differences |
P Value |
|
|
Mean Difference |
Std. Deviation |
|||||
|
GI baseline |
20 |
1.48 |
0.42 |
1.33 |
0.32 |
<0.001** |
|
GI 3 weeks |
20 |
0.14 |
0.12 |
|||
|
Micronuclei baseline |
20 |
1.6 |
2.62 |
-3.55 |
1.63 |
<0.001** |
|
Micronuclei 3 weeks |
20 |
5.15 |
3.03 |
|||
|
Number of micronuceated cells baseline |
20 |
0.75 |
1.3 |
-2.1 |
1.29 |
<0.001** |
|
Number of micronucleated cells 3 weeks |
20 |
2.85 |
1.73 |
|
Parameters |
N |
Mean |
Std. Deviation |
Paired Differences |
P Value |
|
|
Mean Difference |
Std. Deviatio n |
|||||
|
GI Baseline |
20 |
1.65 |
0.46 |
1.52 |
044 |
<0.001** |
|
GI 3 weeks |
20 |
0.12 |
0.12 |
|||
|
Micronuclei baseline |
20 |
2.55 |
3.38 |
-0.55 |
0.60 |
<0.001** |
|
Micronuclei 3 weeks |
20 |
3.1 |
3.16 |
|||
|
Number of micronuceated cells baseline |
20 |
1.4 |
1.67 |
-0.6 |
0.68 |
<0.001** |
|
Number of micronucleated cells 3 weeks |
20 |
2 |
1.84 |
|
|
Chlorhexidine (n=20) |
Listerine (n=20) |
P Value |
||
|
|
Mean |
SD |
Mean |
SD |
|
|
GI Baseline |
1.48 |
0.42 |
1.65 |
0.46 |
0.23 |
|
GI 3 weeks |
0.15 |
0.12 |
0.13 |
0.12 |
0.61 |
|
GI Difference |
1.34 |
0.32 |
1.52 |
0.44 |
0.13 |
|
Micronuclei baseline |
1.60 |
2.62 |
2.55 |
3.38 |
0.33 |
|
Micronuclei 3 weeks |
5.15 |
3.03 |
3.10 |
3.16 |
0.04* |
|
Micronuclei difference |
3.55 |
1.64 |
0.65 |
0.49 |
0.001** |
|
Number of micronuceated cellsbaseline |
0.75 |
1.3 weeks |
1.40 |
1.67 |
0.166 |
|
Number of micronucleated cells 3 weeks |
2.85 |
1.73 |
2.00 |
1.83 |
0.14 |
|
Number of micronuceated cellsdifference |
2.10 |
1.29 |
0.70 |
0.57 |
0.001** |
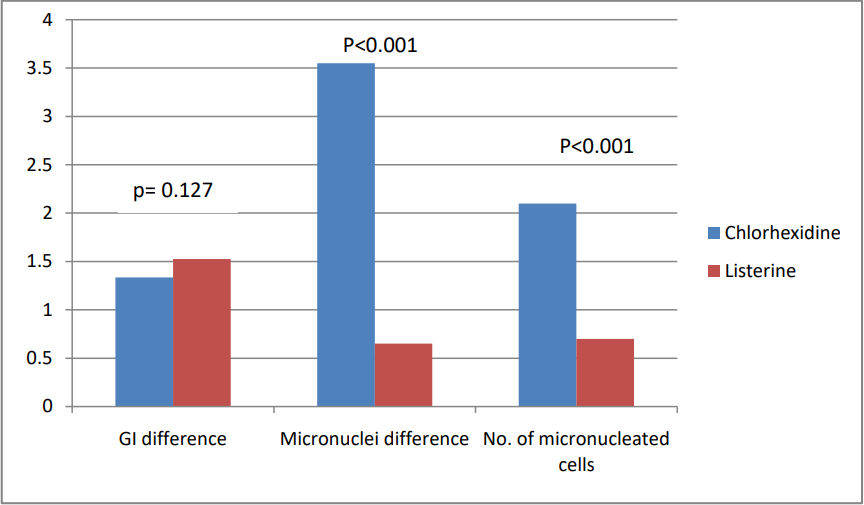




Discussion
Mouthwashes are used routinely as an adjunct to scaling and root planing. Chlorhexidine mouthwash is known as gold standard while Listerine is a widely used essential oil mouthwash. CHX is a chemical agent currently used as a local antiseptic[6], [7] in daily clinical practice, but as certain studies shows cell damage with the overuse of CHX,[1] the cytotoxicity of CHX has been assayed in the present study along with a comparison of cytotoxicity of Listerine Mouthwash to assess that which one is safer for long term use for plaque control.
To assess cytotoxicity buccal epithelial cells are taken as samples because, exfoliated epithelial cells constitute biomarkers of cytotoxicity; using a simple diagnostic assay, the response of the target tissue to the action of carcinogens can be revealed without the need for cells to be maintained in vitro.[8]
In the present study PAP stain method was used. A study to compare Papanicolaou’s (PAP) and May Grunwald’s Giemsa (MGG) staining techniques which are done to detect micronuclei (MN) in exfoliated buccal mucosal cells. They concluded that PAP is a better stain as compared to May Grunwald’s Giemsa (MGG) for counting micronuclei.[9], [10]
The effect of CHX on buccal cells was studied which has shown an increase in damaged cells. It was suggested that this damage could occur before DNA repair begins and may be reversible. The study also indicated that DNA damage could be caused by even small amounts of absorbed mouthwash. The abnormalities observed in oral cell samples reflect underlying chromosomal issues.[1] Therefore, the impact of mouthwash exposure can be shown by monitoring cell damage over time. Therefore, monitoring the cytotoxic exposition of this tissue can reflect the damage that has occurred in 3 weeks. Also, 3 weeks for follow-up was taken because in a Cochrane database of systematic reviews stated that there is high-quality evidence that the use of mouth rinses containing Chlorhexidine mouthwash in addition to usual tooth brushing and cleaning for 4 to 6 weeks or 6 months leads to a large reduction in the build-up of plaque. Rinsing for 4 weeks or longer causes tooth staining, temporary taste disturbance etc.[11] Hence, to avoid staining of tooth surface and altered taste sensation-like problems; participants were advised to use the mouthwash for 3 weeks, and at the end of the 3rd week follow-up for cytotoxicity of buccal cells was taken.
While a study was done to evaluate the genotoxic effect of Chlorhexidine mouthwash concluding that CHX mouthwash is cytotoxic to buccal epithelial cells and there is an incremental trend in cytotoxicity as the duration of usage is increased. The incremental trend was assessed from < 1 week to 24 weeks period, with maximum number of micronuclei found at 24 weeks. This finding suggest that prolong use of Chlorhexidine mouthwash should be avoided.[12]
Listerine typically contains essential oils such as thymol, eucalyptol, menthol, and methyl salicylate. These oils contribute to its antimicrobial properties and freshening effect, hence LN is used as part of daily oral care to reduce bacterial plaque. Its essential oil contents are known to be effective for controlling periodontal diseases. During recent years, more basic research and clinical investigations have been carried out to evaluate the LN toxicity on different experimental models.
A study assessed the relative toxicity of mouth rinses including Chlorhexidine mouthwash, triclosan, essential oils in ethanolic solution (LN). The results of the mentioned study indicated that, the toxicity of LN showed no significant difference compared to other mouth rinses i.e. triclosan, saline and CHX,[13], [14] which is in contrast to this study.
Another study was done to evaluate the cytotoxicity after Listerine use, stated that there were no statistically significant differences (p > 0.05) between the control group (Where mouthwash is not used) and the groups treated with LN alone in both analyzed endpoints.[15]
While, the clinical and cytological levels, neither alcohol-based (Listerine) nor alcohol-free (CHX) mouth rinses cause statistically or physiologically significant negative reactions, which is also in contrast to the results of present study.[16] These differences in the results could be due to different methods of cytotoxicity on buccal epithelial cells. A uniform technique for cytological evaluation can be implemented to ensure accurate outcomes.
Conclusion
After 3 weeks of use, both types of mouthwash reduced gingival inflammation, but Chlorhexidine mouthwash showed a higher increase in micronuclei and micronucleated cells compared to Listerine. This suggests greater cytotoxicity with Chlorhexidine mouthwash. Larger randomized trials are necessary to thoroughly compare the genotoxic effects of various commercial mouthwashes for better guidance in oral care practices.
Source of Funding
None.
Conflict of Interest
None.
References
- K Eren, N Özmeriç, S Şardaş. Monitoring of buccal epithelial cells by alkaline comet assay (single cell gel electrophoresis technique) in cytogenetic evaluation of chlorhexidine. Clin Oral Investig 2002. [Google Scholar]
- A Santos. Evidence-based control of plaque and gingivitis. J Clin Periodontol 2003. [Google Scholar]
- A Wagh, J Raval, R G Aiyer, S Amin. Micronuclei in exfoliated oral epithelial cells in tobacco users and controls with various oral lesions: A study from Gujarat, India. Indian J Otolaryngol Head Neck Surg 2019. [Google Scholar]
- EO Erdemir, A Şengün, M Ülker. Cytotoxicity of mouthrinses on epithelial cells by micronucleus test. Eur J Dent 2007. [Google Scholar]
- PE Tolbert, CM Shy, JW Allen. Micronuclei and other nuclear anomalies in buccal smears: A field test in snuff users. Am J Epidemiol 1991. [Google Scholar]
- FP Deus, A Ouanounou. Chlorhexidine in dentistry: Pharmacology, uses, and adverse effects. Int Dent J 2022. [Google Scholar]
- ZLS Brookes, R Bescos, LA Belfield, K Ali, A Roberts. Current uses of chlorhexidine for management of oral disease: a narrative review. J Dent 2020. [Google Scholar]
- N Holland, C Bolognesi, M Kirsch-Volders, S Bonassi, E Zeiger, S Knasmueller. The micronucleus assay in human buccal cells as a toolfor biomonitoring DNA damage: the HUMN project perspective on current status and knowledge gaps. Mutat Res 2008. [Google Scholar]
- S Palaskar, C Jindal. Evaluation of Micronuclei Using Papanicolaou And MayGrunwald Giemsa Stain In Individuals With Different Tobacco Habits - A Comparative Study. J Clin Diag Res 2010. [Google Scholar]
- FL Kitchen, CM Cox. . Papanicolaou Smear. StatPearls [Internet] 2022. [Google Scholar]
- P James, HV Worthington, C Parnell, M Harding, T Lamont, A Cheung. Chlorhexidine mouthwash mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst Rev 2017. [Google Scholar]
- S Khan, A Khan, S Hasan. Genotoxic assessment of chlorhexidine mouthwash on exfoliated buccal epithelial cells in chronic gingivitis patients. J Indian Soc Periodontol 2016. [Google Scholar]
- Ros-Llor I, P Lopez-Jornet. Cytogenetic analysis of oral mucosa cells, induced by Chlorhexidine mouthwash, essential oils in ethanolic solution and triclosan mouthwashes. Environ Res 2014. [Google Scholar]
- V Garaj-Vrhovac, D Zeljezic. Evaluation of DNA damage in workers occupationally exposed to pesticides using single-cell gel electrophoresis (SCGE) assay. Mutat Res Genet Toxicol Environ Mutagen 2000. [Google Scholar]
- H Türkez, B Togar, T Arabaci. Evaluation of cytotoxicity after application of Listerine® on human lymphocytes by micronucleus and single cell gel electrophoresis assays. Toxicol Ind Health 2012. [Google Scholar]
- V Pant, V Gupta, S Pandey, A Pant. Efficacy and safety evaluation of alcohol-containing and alcohol-free mouth rinses: A clinicocytological study. J Indian Soc Periodontol 2021. [Google Scholar]
